Big Lies by Big Tobacco
Mi-Pod Wholesale Customers, vapers, convenience store owners, and concerned citizens should be aware that Altria and RJ Reynolds have launched a nationwide campaign to pass PMTA Registry Laws at the state level. Using their considerable lobbying power and financial resources, these laws have been introduced in dozens of states.
PMTA is an acronym for Premarket Tobacco Application. This is the horribly broken FDA process that has approved all of seven obsolete cigalikes and 23 SKUs, which includes a narrow selection of unpopular artificial tobacco inspired flavor profiles.
This process threatens to deny adult vapers access to the products they prefer. Especially hard hit are marginalized groups. This is why the Center for Black Equity called on the FDA to approve a wide range of e-cigarettes to ensure Black and LGBTQIA+ Americans can access effective options.
A bipartisan group of US Senators have questioned the failing PMTA process, which faces and will continue to face numerous court challenges.
The PMTA Process was deemed busted beyond repair in a decision by the Fifth Circuit Court of Appeals in the case WAGES AND WHITE LION INVESTMENTS v. FOOD DRUG ADMINISTRATION. They summarized the entire regulatory debacle with the following memorable quote:
Altria's Past Attacks PMTA Process
In fact, Altria themselves questioned the PMTA process in the past. It is interesting that they are in support of the FDA's flawed and incomplete application process now that it can be used to their advantage. Their PMTA Registry Bills will remove products popular with adults from the market but are structured so their products remain on the shelves.
Here are some of Altria's past statements on the PMTA process.
- Unfortunately, Center for Tobacco Products (CTP) appears to be relying on a “fatal flaw” analysis to reject applications. This approach has drawn legal challenges, fuels suspicion that CTP is adjudicating applications with a pre-determined agenda and ultimately undermines the credibility of the Center and the Agency. Further, without a full and complete review, manufacturers lack insight needed to address deficiencies and re-submit an improved application.
- Science-based engagements should be encouraged between CTP and applicants. Yet, CTP project managers and Office of Science staff appear hesitant to have substantive engagements.
- Unfortunately, how CTP is currently interpreting and implementing that standard leaves manufacturers guessing as to what scientific evidence and rationale will satisfy APPH scrutiny. Without a clearly articulated definition for “appropriate for the protection of public health,” CTP risks making arbitrary and inconsistent decisions and undermining innovative product development, research execution and, ultimately, tobacco harm reduction.
Mi-Pod and PMTAS
Mi-Pod has submitted timely PMTA applications that are still pending with FDA; they have not been refused or denied. We are also opposed to PMTA Registry Laws which are written by the tobacco industry to remove any remaining competition they face on the open market.
The FDA has NOT granted final authorization to Big Tobacco’s most popular vaping products either, such as the Vuse Alto, which is why PMTA Registry Bills are invariably worded so that only products on the market eight years ago (2016) can be sold. Click on the CASAA Banner above to view their most recent calls-to-action and information on impending vape bans.
Flavor Bans Increase Cigarette Sales
PMTA Registry Laws have been backed by tobacco lobbyists in thirty-four states. If passed, the adult vaping consumer will either pay more to vape or be funneled back onto combustible cigarettes, either through the sheer expense Big Tobacco's pod kits or the lack of the adult appealing flavors that the majority of vapers prefer.
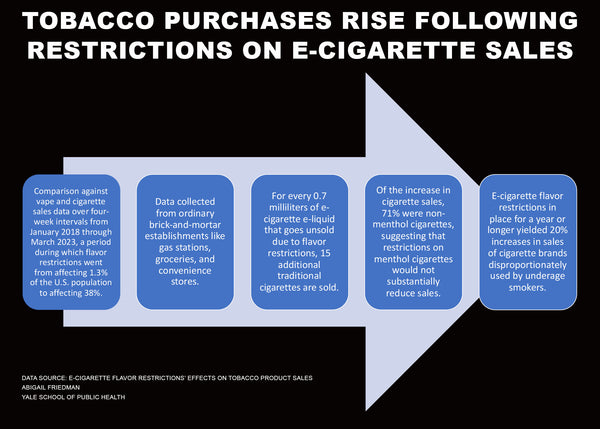
A recent FDA sponsored study found that flavor bans increase cigarette sales. Big Tobacco's prefilled pods are limited to tobacco flavors. Cigarettes of course are not impacted by these restrictions. Their motivations for supporting PMTA Registry Bills are not difficult to discern.
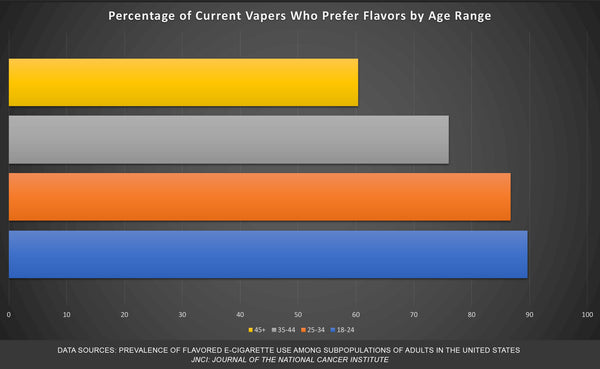
Regardless of age, adult vapers overwhelmingly prefer the characterizing flavors that the FDA seems so reluctant to approve. This benefits the tobacco industry. Even the 45+ age cohort prefers flavored vapes at a rate of over 60 percent. This despite flavor bans limiting adult access to in many areas of the country.
Consumers Pay for Big Tobacco Monopoly
There are two reasons why prefilled pods made by Big Tobacco are hemorrhaging sales to disposable vapes. The first is that prefilled pods are only available in artificial tobacco flavors, not the popular fruit and beverage inspired flavors that can be found in every single consumable product on the market.
Products ranging from kombucha to hard seltzer feature artificial flavor profiles that are very similar to those which are vilified when used in popular vape juices. It is almost impossible to imagine an adult appealing consumable product anchored on the cloying notes of fauxbacco. Possibly a coffee blend? It is a very short list.
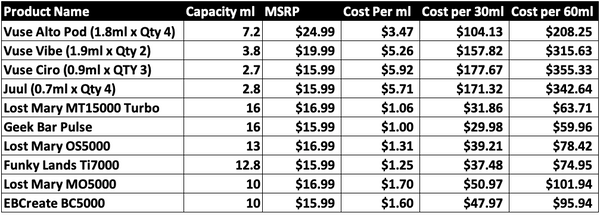
Then there is the price issue. The difference in cost per 30ml of e-liquid vaped is massive. The consumer is not the only beneficiary of fair competition. Products from the independent vape industry have much better margins for the wholesaler.
No editorializing or studies are needed here. It costs over $200 to vape 30ml of e-liquid from a Vuse Alto and $60 from a Lost Mary. Of course the companies trying to sell e-liquid at two hundred dollars per 30ml want rival products banned.
Big Tobacco’s Motivation for PMTA Registry Laws
The root of the big PMTA Registry push can be found in a Barclay’s Earnings Report Guidance for Altria. Not every big tobacco giant is pursuing these serious restrictions on adult vapers. But after consecutive quarters where revenue expectations were not met, Altria has decided eliminating the competition is the easiest solution. 
To gain support for their efforts to put Americans out of work and drum up cigarette sales, Big Tobacco has turned to telling big lies about the vaping industry and American businesses that simply seek a level playing field.
But it is not all bad news for the tobacco industry, former FDA Chief Scott Gottlieb noted that declines in smoking have been effectively checked by the flavor bans and restrictions placed on vaping products. Even if MyBlu or Vuse are losing sales to disposable vapes, their legacy combustible products remain a cash cow that dominate shelf space and market share.
Declines in smoking rates have started to stall ; if we're going to help more adults quit combustible cigarettes we have to embrace harm reduction and offer adult smokers modified risk nicotine products that don't have same risks as combusting tobacco 1/2 https://t.co/l3a1zmffqc
— Scott Gottlieb, MD (@ScottGottliebMD) February 26, 2024
The Future of Cigarette Sales
Smoking rates have been declining for almost 60 years. This progress has been checked by FDA regulations. An FTC report in the October 2021 found cigarette sales increased for the first time in a quarter century.
Even with this recent reversal of fortune, the erosion of the core cigarette business has been severe. As reported in the Wall Street Journal:
"According to data from Marlboro maker, cigarettes’ share of the U.S. nicotine industry fell to 60% last year, down from 80% in 2018. Smokers are switching to smoke-free products such as vapes in higher numbers than expected. If the trend continues, it will only take another three years for cigarettes’ share to slip below 50%."
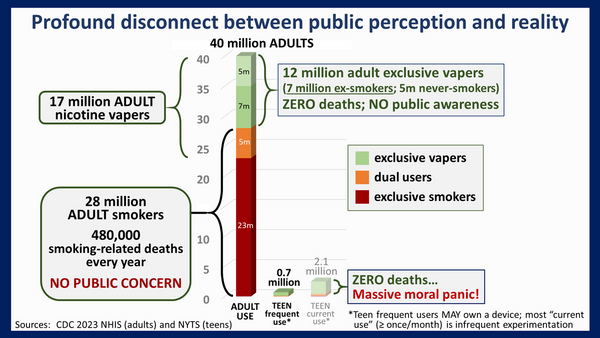
The table below shows where this pressure has been coming from. The US adult population has grown by some 23 million since 2011. The total number of adult nicotine users, be it tobacco, vapes or pouches, has declined.
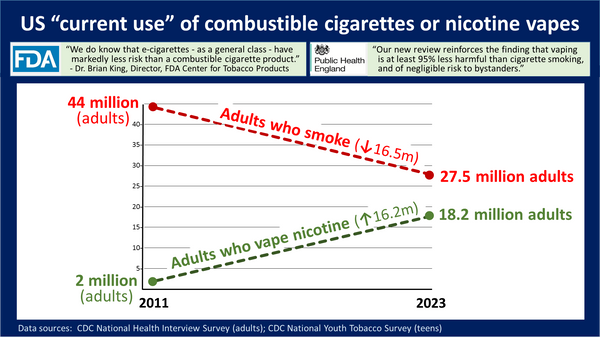
The CDC reported in 2023 that e-cigarette use continues to decline among high school students. The easiest way for the tobacco industry to prevent a further depletion of their customer base is to eliminate the competition through regulatory capture or PMTA Registry laws that create a de facto ban on competing products.
Who is Mi-Pod?
Mi-Pod is a minority owned business that has 80 employees in Arizona. It was awarded the #1 Top Workplace in Arizona for 2023. Mi-Pod’s mission is to ‘eradicate the harms caused by smoking’. That mission remains our focus. We will pursue our goal in the face of insult and slander from the tobacco companies and their lobbyists. We will continue to call them out on their lies and misinformation.

We will resist their use of high-powered lobbyists to destroy small and medium-sized businesses with legal fees and frivolous lawsuits. Their policy system “outspending” those with the temerity to challenge Big Tobacco has proven effective for over a century.
Big Tobacco, Big Lies
Science is clearly not on the side of PMTA Registry laws, which are vape flavor bans that also bar countless popular products.
In late February, a New England Journal of Medicine study stated that we have reached a scientific tipping point when it comes to vaping.
A University of Massachusetts Amherst study this winter found that vaping helps more adults quit smoking than nicotine replacement therapies (NRT) such as the patch, gums, and lozenges. Past studies, including a gold-standard and peer reviewed work published in the New England Journal of Medicine, found that vaping is a much more effective cessation tool than Big Pharma manufactured nicotine replacement therapies.
A more enlightened approach would more closely mirror the National Health Service in the United Kingdom, which hosts the resource page Vaping to Quit Smoking.
During their relentless lobbying, the Altria took note that Mi-Pod was fighting hard for adult access to vapes and this was at cross purposes with their desire to limit adult freedoms and use regulatory capture to defend market share.
The document below is an actual picture of the crude and inaccurate document their lobbyists have been handing out to politicians.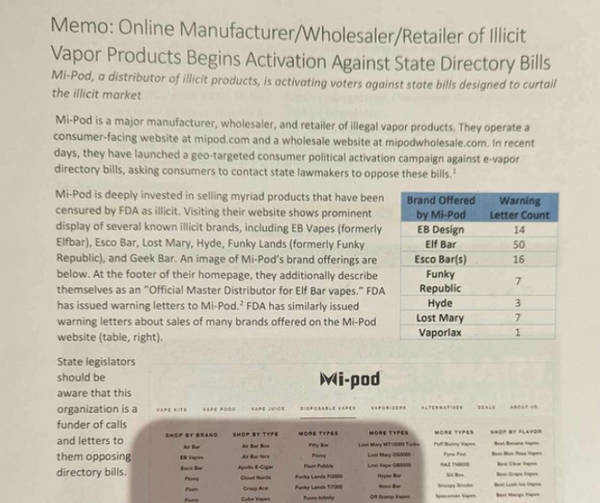
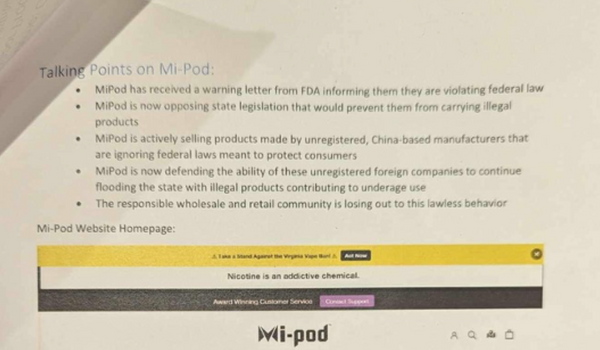
Lie #1: Mi-Pod is a Manufacturer and Reseller of Illicit Vape Products
Mi-Pod does not sell counterfeit vapes, and only work with legitimate manufacturers who we know are working through the PMTA process and have active PMTAs pending with FDA. Check out our feature article, How to Spot a Fake Vape, for more information.
Lie #2: Vape Products Not Manufactured by the Tobacco Industry are Illicit and Illegal
The term ‘illicit’ or ‘illegal’ is false and misleading. The vape products of RJ Reynolds, save for a few obsolete models, have not received marketing granting orders (MGOs) either. RJ Reynolds products were actually denied by FDA, and they sued the FDA to have the denial overturned.
To quote British American Tobacco (BAT), the parent company of RJ Reynolds:
“This decision flies in the face of proven science and is contrary to the FDA’s stated goal of reducing the health effects of tobacco use. This disappointing decision would harm, not benefit, public health. We believe appropriately regulated flavoured vaping products- including menthol- are critical in supporting adult smokers migrate from combustible cigarettes.”
To date, the FDA has approved seven different vaping products. They are all obsolete designs. Altria does not even bother selling one of the approved products, the Vuse Ciro.
Lie #3: Warning Letters from FDA Show Mi-Pod is a Bad Faith Actor
A warning letter is just that, a “warning”, and a request for more information. They do not represent a penalty or judgement. The warning letter Mi-Pod received was for SV Cigs. This was a legacy cigalike product that had been on the market since 2008. It was serving older customers, as the average buyer was over 55 years of age. This product had been removed from sale at the time of the letter because it represented less than 0.1% of sales, and it was cost prohibitive to spend millions to file a PMTA for those older products. The FDA was satisfied by the remedy.
Lie #4: Mi-Pod is Selling Products from Unregistered China-based Manufacturers
This is a red-herring. Mi-Pod only sells products from factories and suppliers that we have met with, visited, and can provide testing data, certificates of analysis (COAs), and use GMP Manufacturing standards.
RJR and NJOY Altria products are MADE IN CHINA with components from THE SAME factories (Smoore) that make the components for popular disposable vape brands (ex: Heaven Gifts). To call our products illegal or illicit and ignore that their products use components from the same facilities is pure fear-mongering.
Below is a screen grab from Vuse's own website. Note that their products are from China.

Altria's proprietary e-liquids are only "blended" in the United States. They cannot claim these e-liquids are made in the USA because the ingredients are primarily from China.
Altria’s NJoy Ace was designed as a CANNABIS product and sold in USA before NJOY began using as nicotine. There are THC compatible pods for the NJOY on the market.
Lie #5: Mi-Pod is Irresponsible and Promotes ‘Lawless Behavior’
As described in their own words, Altria and RJR are actively trying to undermine the reduction in smoking and will stop at nothing to protect their own bottom line.
Barclays Analysis of Altria’s business shows they are only able to hit targets if they can stop disposables and increase sales of combustible cigarettes. These hypocrites have no ground to stand on when it comes to youth initiation – they have always been the culprits.
Even the FDA’s own ‘sting’ operations find that the violations in selling to underage persons happen mostly in convenience stores and gas stations where Big Tobacco's vape products are predominantly sold.
PMTA Registry Bills bills do NOTHING to stop the “straw buyers” which are the main source of youth-access, nor do the bills stop truly illegal products which are black market clones and small cheap brands that are not sold by Mi-Pod or other legitimate distributors.
Altria is even funding ‘hit-pieces’ with false information like the article in Floridapolitics.com by Peter Schorsch.
Let's take a closer look at @PeterSchorschFL and @Fla_Pol who trafficked a completely erroneous hit piece about us this week. It's a wild ride that includes house arrest, check bouncing, pay-for-play journalism, FEC violations, and a national media ethics exposé.
— American Vapor Manufacturers (@VaporAmerican) February 29, 2024
THREAD 🧵 pic.twitter.com/Qqf34mQfbp
THE BIG LIE: Mi-Pod Promotes Underage Vaping
There is no greater calumny and insult than accusing Mi-Pod of endorsing or benefiting from underage vaping. Youth vaping remains an existential threat to the independent vaping industry. Our products are designed and meant for adults who already vape or smoke. We have no interest in selling to minors and are not interested in courting adults who are not currently using nicotine products.
The market of smokers who do not currently vape is quite large enough, thank you. And all we ask is for a level field of play.
Youth vaping rates have fallen 60 percent since 2019, and it is no accident that dishonest advocates of the Hawaii’s 2024 Vaping Restrictions (HB1778) cited 2016 to 2019 youth tobacco use patterns to prove the necessity of this ordinance.
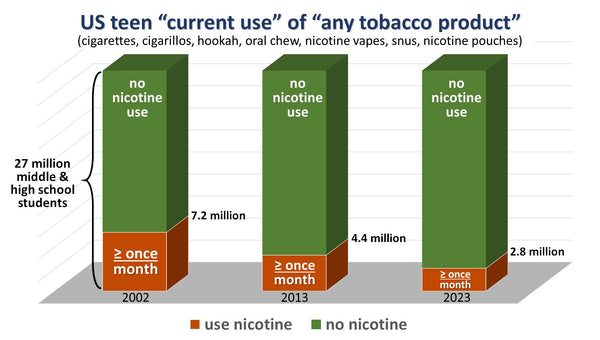
Bad actors selling nicotine to minors cost thousands of adults their jobs, destroyed countless business and the fallout has denied millions of adults access to the ash and smoke free tobacco alternative products they prefer.
It defies credulity that Altria, who purchased Juul at the height of their popularity with underage vapers in December 2018 for 12.8 billion and later sold this tanked asset for scraps of IP, would level this accusation.
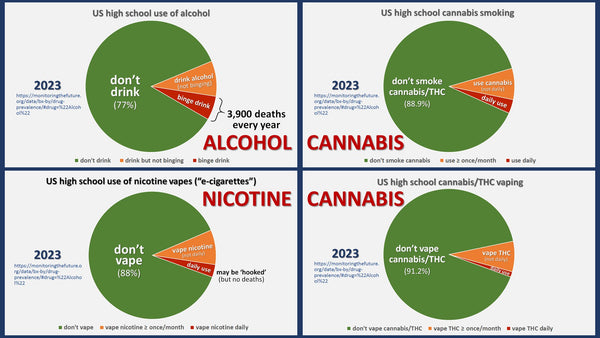
Mi-Pod does not promote or contribute to ‘youth’ use. Besides youth tobacco/nicotine use being at ALL-TIME lows nationally, and our owner having 3 teenage kids of his own to worry about, everything we do from marketing, product selection and language is guided by limiting youth exposure and protecting adult access.

Our websites use Blue-Check 3rd party age verification, adult-signature on delivery, and our B2B wholesale site requires business and tobacco license authentication to be able to purchase.
Fact: Mi-Pod Opposes PMTA Registry Laws
Of course, Mi-Pod opposes bills that would annihilate the independent vaping industry, put thousands out of work, and funnel adult vapers back onto combustible cigarettes. These laws were written by Altria lobbyists in conjunction with RJR to create a duopoly in the market and protect their cigarette sales.
PMTA Registry Bills creates an unfunded mandate that turns the state into an enforcement arm for failed FDA policies, policing any products not manufactured by Big Tobacco.
PMTA Registry laws are a confluence of fear mongering and outright lies. The PMTA FDA system is broken (capricious, politicized, and illegal according to recent court decisions overturning MDOs). They do not represent the best interests of public health.
As described above, RJR’s parent company BAT and Altria actively state that the FDA process is broken and doesn’t reflect the science. The Fifth Circuit Court of Appeals had an even more damning take. Wages and White Lions Investments, L.L.C. (DBA Triton Distribution) v. the Food & Drug Administration is replete with forceful language criticizing the FDA's unusual schemes, deceitfulness, and the grievous consequences it has triggered.








Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.